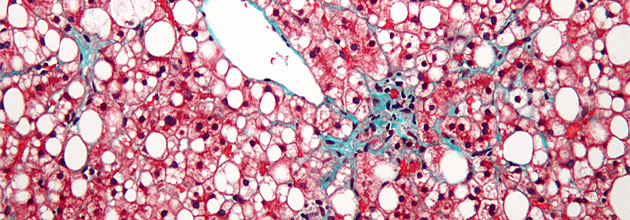
In an April 23 conference update, analyst David Sherman of LifeSci Capital commented on the results of DURECT Corp.'s (DRRX:NASDAQ) Phase 1b study of DUR-928, noting the compound "was well tolerated overall," that the data support ongoing evaluation of the therapy, and that "investigators speculated on the plausibility of a therapeutic effect due to biomarker reductions at approximately 12 hours following dosing."
In an April 25 Laidlaw & Company research report, analyst Francois Brisebois observed that at its lowest dose, DUR-928 "showed reduction in liver function and injury biomarkers after only 12 hours. Additionally, inflammation markers and mediators such as high sensitivity C-reactive protein (hsCRP) and IL-18, as well as CK18 (cell death biomarker), were reduced in NASH (especially in cirrhotics). The full data set confirmed these findings with a single dose, which is a real positive in our opinion."
The Laidlaw analyst also noted that with the Phase 3 PERSIST trial "of Posimir for post-operative pain expected to read-out by year end-2017, IND [investigational new drug] filings in both liver and kidney programs around mid-2017 and initiation of a Phase 2 trial for DUR-928 in mid-2017 for primary sclerosing cholangitis (PSC), 2017 should be an exciting year for DRRX."
According to a report by Reuters, the market for NASH, also known as nonalcoholic fatty liver disease, "is forecast to be $20 billion to $35 billion as populations with fatty diets increasingly fall victim to a condition with no approved treatments."
Large pharmas including Pfizer Inc. (PFE:NYSE) and Bristol-Myers Squibb Co. (BMY:NYSE) are looking for candidates for development and marketing, according to the Reuters article, which quotes Morris Birnbaum, chief scientific officer for internal medicine for Pfizer, as saying the company is "actively looking on the outside for opportunities . . .to complement our internal program."
The Reuters article also quoted Len Yaffe of StockDoc Partners, who noted the DURECT drug "looks incredibly promising as it relates to inflammation and fibrosis." Yaffe told the news agency that "investors with tolerance for risk could do well to buy shares in several small-cap and micro-cap companies with promising NASH drugs in early development . . .the payoff could be considerable."
Read what other experts are saying about:
Want to read more Life Sciences Report articles like this? Sign up for our free e-newsletter, and you'll learn when new articles have been published. To see recent articles and interviews with industry analysts and commentators, visit our Streetwise Interviews page.
Disclosure:
1) Tracy Salcedo compiled this article for Streetwise Reports LLC and provides services to Streetwise Reports as an independent contractor. She or members of her household own shares of the following companies mentioned in this article: None. She or members of her household are paid by the following companies mentioned in this article: None.
2) The following companies mentioned are sponsors of Streetwise Reports: DURECT Corp. Streetwise Reports does not accept stock in exchange for its services. Click here for important disclosures about sponsor fees. The information provided above is for informational purposes only and is not a recommendation to buy or sell any security.
3) Comments and opinions expressed are those of the specific experts and not of Streetwise Reports or its officers.
4) The article does not constitute investment advice. Each reader is encouraged to consult with his or her individual financial professional and any action a reader takes as a result of information presented here is his or her own responsibility. By opening this page, each reader accepts and agrees to Streetwise Reports' terms of use and full legal disclaimer. This article is not a solicitation for investment. Streetwise Reports does not render general or specific investment advice and the information on Streetwise Reports should not be considered a recommendation to buy or sell any security. Streetwise Reports does not endorse or recommend the business, products, services or securities of any company mentioned on Streetwise Reports.
5) From time to time, Streetwise Reports LLC and its directors, officers, employees or members of their families, as well as persons interviewed for articles and interviews on the site, may have a long or short position in securities mentioned. Directors, officers, employees or members of their immediate families are prohibited from making purchases and/or sales of those securities in the open market or otherwise from the time of the interview or the decision to write an article, until one week after the publication of the interview or article.
Additional disclosures about the sources cited in this article